WHAT IS A CLINICAL PROTOCOL?
A Clinical History taking and examination protocol is a document that outlines the methods to conduct a complete clinical case evaluation. The protocol must be carefully designed to safeguard so that the outcome safeguards the health and safety interest of the participants i.e. Evidence Based Medicine/ patients, as well as gathers as much data to as understand patient specific data fully understand the patient’s condition needed for management of the case by a remote doctor. It should provide the following salient features :
...
| Info |
|---|
A clinical protocol should have:
|
...
|
...
|
...
|
...
|
...
|
...
All of the above ensures that the final |
...
case data would be clear, extensive yet concise and provide enough details for all those involved in the study to use it. |
HOW TO BUILD A CLINICAL PROTOCOL?
In order to build a clinical protocol, one has to start thinking about how a doctor would think. For example, when a patient comes in a clinic/ OPD setting, the doctor would first proceed to a checklist that is already in his mind which has molded over years of medical schooling and his or her own experience and skill set. Now, this is a very subjective checklist and may vary between different sets of doctors and also between individual doctors.
Thus to have an objective, robust and medico-legally risk-free system we need to have a clinically correct, personalized, up-to-date and reliable scientific evidence , system. Also, for the same reasons as above, clinicians prefer the knowledge base provided by clinical practice guidelines or protocols and recommendations that is compiled systematically by experts in specific areas of therapymedicine. These systems provide an Evidence-Based Approach to Clinical Case Evaluation and are therefore aptly termed as Evidence Based Medicine.
...
HOW TO USE EVIDENCE-BASED RESEARCH TO BUILD A PROTOCOL?
For
...
an evidence-based approach to writing clinical protocols, we need
...
certain guidelines. These guidelines are basically literature in the latest editions of internationally acclaimed medical textbooks (gold standard), research articles in journals (national and international), local disease trends and demographics, etc. While this dogged search for guidelines and answers is not the quickest, it is the cornerstone of good care.
| Info |
|---|
Gold standard medical books include, not in any order, Davidson's Principles and Practice of Medicine, Harrison's Principles of Internal Medicine, Mc Leods Guide to physical examination, Gray’s Anatomy, Robbins and Cotran Pathological |
...
Basics of Disease, Guyton and Hall, Textbook of Medical Physiology, etc. You can find a more extensive list here. (ADD LINK) |
A review article is a piece of literature that summarizes the current and popular understanding on of a topic.A review article also surveys and summarizes previously published studies, rather than reporting new facts or analysis. Review articles are sometimes also called survey articles or, in news publishing, overview articles. Medical academic publications that specialize in review articles are known as Medical review journals. Some accepted journals, worldwide,
| Info |
|---|
Internationally acclaimed journals include BMJ, NEJM, The Lancet, JAMA, etc. |
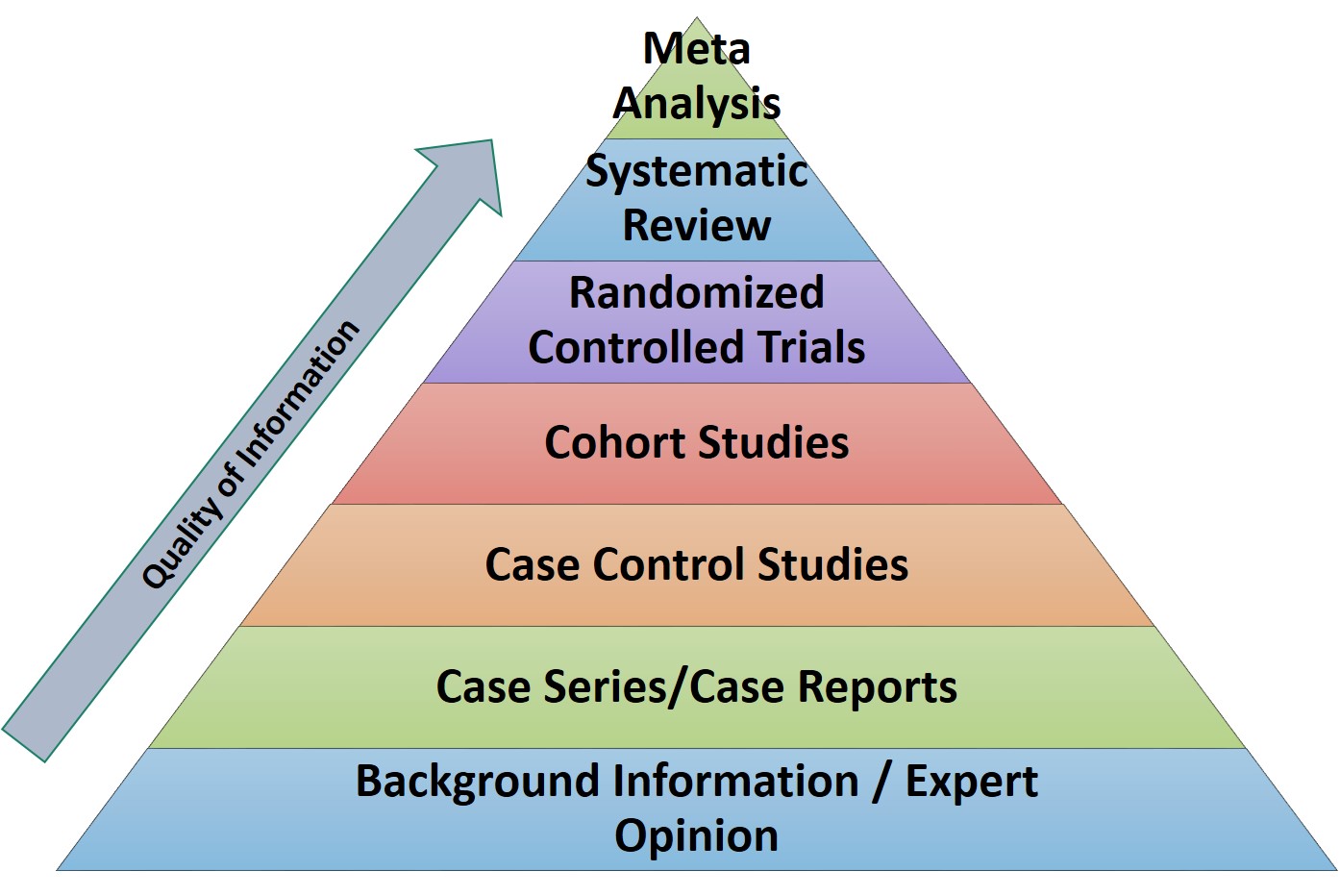
In research articles, there are different types of studies which denote the quality of the article. Another way of gathering clinically relevant data is via For example, Meta-Analysis and Systematic Reviews are the gold-standard of clinical information. They summarise the findings of several studies on a particular subject.
| Info |
|---|
Best study designs in the order of preference are shown as follows |
Other ways of gathering Evidence based medical data:
Online libraries such as PubMed (Central), Scopus, Medline, Cochrane and Google scholar, besides others. These sometimes have a subscription fee for their platform which can be borne by self or by
...
institutions one is a part of.
...
Online content from internationally prominent and renowned organisations such WHO, AHRQ, IOM, AMA, IMA, MOHFW, etc. can also be used and is a practice well accepted while researching and citing articles.
Even in research articles, there are different types of studies which denote the quality of the article. For example, Meta-Analysis and Systematic Reviews are the gold-standard of clinical information. They summarise the findings of several studies on a particular subject. The others in decreasing order of preference is given in the pictorial representation below.
Evidence Evidence-based research must be backed by peer reviews. Therefore research papers or articles of various subjects always include citation or references. While writing a research paper, it is always important to give credit and cite your sources; this . This lets you acknowledge others’ ideas and research you’ve used in your own work and to prove your work is based on/ has the backing of certain standard guidelines and to avoid plagiarism. Plagiarism occurs when you borrow another's words (or ideas) and do not acknowledge that you have done so. It is unethical and a very serious offense.